Background: Most patients with lower-risk myelodysplastic syndrome (LR-MDS) develop anemia and a high proportion of patients become dependent on red blood cell (RBC) transfusions. MDS-related anemia is associated with low hemoglobin (Hb) levels, iron overload and poor quality of life. Erythropoiesis-stimulating agents and other drugs, including lenalidomide, luspatercept and imetelstat (an investigational product), can lead to RBC transfusion independence and improve Hb levels in many patients; however, responses are generally transient and novel treatments are needed for this population.
Etavopivat is an investigational, once-daily, selective erythrocyte pyruvate kinase (PKR) activator in development for the treatment of sickle cell disease (SCD) and anemia in patients with LR-MDS. In a phase 1 study in adult and adolescent patients with SCD by Saraf et al. HemaSphere. (2022), etavopivat 400 mg once daily was considered well tolerated and showed sustained Hb increases up to 12 weeks for most patients. Additional studies in healthy volunteers and patients with SCD support an acceptable safety profile together with improved RBC health and function during continuous treatment with etavopivat.
In patients with LR-MDS, ineffective hematopoiesis may be due, in part, to insufficient ATP production, leading to increased erythroid lineage apoptosis. By activating PKR, etavopivat may improve RBC health and function by stimulating energy production (increasing ATP levels), increasing Hb levels (decreasing anemia), and decreasing erythroid lineage apoptosis.
Aims:Describe the design of a multicenter, open-label, phase 2 study (FORTITUDE; NCT05568225) evaluating hematologic improvements in patients with very low-, low- and intermediate-risk MDS during treatment with etavopivat.
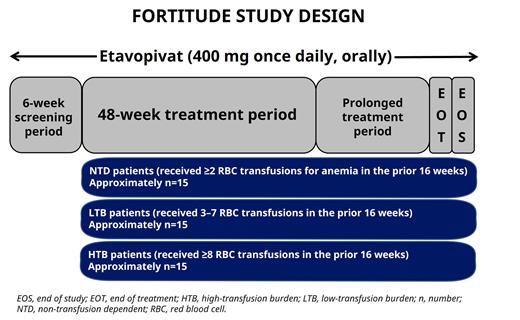
Study design:Key eligibility criteria include age ≥18 years; very low-, low- and intermediate-risk MDS per the Revised International Prognostic Scoring System; and anemia defined by mean Hb <10.0 g/dL and <3 RBC transfusions <16 weeks prior to first dose for non-transfusion-dependent (NTD) patients or ≥3 units of RBCs for anemia <16 weeks prior to first dose for transfusion-dependent (TD) patients. Key exclusion criteria include MDS associated with del 5q cytogenetic abnormality and known TP53 abnormality, history of acute myeloid leukemia, and an absolute neutrophil count <0.5 × 10 9/L. Additional exclusion criteria include prior treatment with azacitidine; decitabine; erythropoietin, other hematopoietic growth factor treatment or lenalidomide within 30 days of Day 1 or anticipated to be required during the study; and luspatercept within 30 days of Day 1 for NTD patients and within 16 weeks of Day 1 for TD patients.
Patients will receive etavopivat 400 mg once daily for at least 48 weeks. Treatment may be continued beyond 48 weeks in patients demonstrating clinical benefit. The primary objective of the study is to assess hematologic improvements based on an erythroid response over any continuous period ≥8 weeks within 24 weeks on etavopivat treatment in patients with LR-MDS. The primary endpoint will be based on the combined incidence of: ≥1.5 g/dL increase in Hb from baseline maintained for ≥8 consecutive weeks for NTD patients; absence of any transfusion for ≥8 consecutive weeks for low transfusion-burden patients; and reduction by ≥50% of RBC units for ≥8 consecutive weeks for high transfusion-burden patients. Safety, tolerability, pharmacokinetic, and pharmacodynamic properties of etavopivat will be evaluated.
FORTITUDE is open for enrollment in Canada, France, Germany, and the USA. Planned enrollment includes approximately 15 patients in each of the three transfusion requirement-based groups (Figure).
Statistical analysis: The primary endpoint (pooled incidence) will be tested with a one-sided Fisher's exact test at the significance level of 0.025 where 0.2 is the assumed null proportion.
Summary:Etavopivat is a novel, investigational, once-daily, selective PKR activator with the potential to improve RBC health and lifespan. This phase 2 study will assess the safety of etavopivat and its impact on Hb levels and RBC transfusion burden in patients with LR-MDS and anemia.
Disclosures
Sekeres:BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kurome: Consultancy, Current holder of stock options in a privately-held company; Geron: Membership on an entity's Board of Directors or advisory committees. Platzbecker:MDS Foundation: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Curis: Consultancy; Geron: Consultancy. Heje Thomsen:Novo Nordisk, Denmark: Current Employment. Wilson:Forma Therapeutics, a Novo Nordisk Company: Current Employment. Renteria:Kymera Therapeutics: Consultancy; Novo Nordisk: Consultancy. Fenaux:Jazz: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; French MDS Group: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal